The outbreak of botulism-like illnesses caused by sketchy cosmetic injections has raised major concerns among state health officials. The counterfeit Botox shots have resulted in the hospitalization of four out of the six affected individuals, indicating the severity of the situation. The affected states, Tennessee and Illinois, have reported these cases to the Centers for Disease Control and Prevention (CDC), prompting the organization to take immediate action.
In response to the outbreak, the CDC is preparing to issue a nationwide alert to inform healthcare professionals about the potential dangers of counterfeit Botox. This alert will serve as a warning to clinicians, urging them to remain vigilant and report any cases of botulism-like illnesses. By raising awareness among healthcare providers, the CDC hopes to prevent further cases and ensure that appropriate measures are taken to address the situation.
While the CDC’s alert is a crucial step in combating the outbreak, many are still seeking more information about the counterfeit Botox and its source. Questions regarding the origins of the sketchy injections remain unanswered, leaving both medical professionals and the public in a state of uncertainty. Efforts are underway to gather more information about the counterfeit product, including its distribution channels and potential risks associated with its use.
The outbreak serves as a reminder of the dangers of counterfeit medications and the importance of ensuring the safety and efficacy of cosmetic procedures. The incident highlights the need for stricter regulations and increased oversight in the cosmetic industry to prevent the circulation of counterfeit products. Additionally, it emphasizes the importance of educating the public about the risks associated with unregulated cosmetic treatments and the importance of seeking reputable providers.
As the investigation into the outbreak continues, health officials and regulatory bodies are working together to identify the source of the counterfeit Botox and take appropriate action to prevent further harm. In the meantime, individuals are advised to exercise caution when seeking cosmetic treatments and to consult with licensed professionals who adhere to stringent safety standards.
Counterfeit Botox poses a significant danger to individuals seeking cosmetic procedures. Unlike genuine Botox, which is a regulated drug product containing controlled quantities of the botulinum neurotoxin, counterfeit Botox is produced without adhering to the necessary safety measures. This means that the quality, purity, and potency of the product cannot be guaranteed.
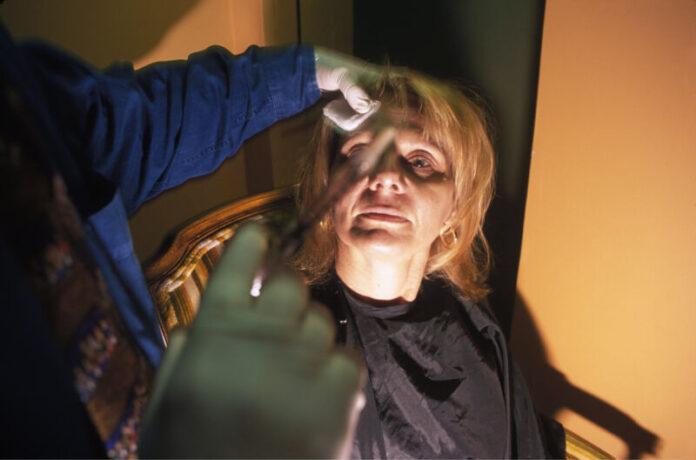
One of the main concerns with counterfeit Botox is the source of the product. In the cases reported so far, it is evident that the injections were administered in non-medical settings, such as homes or cosmetic spas. These settings lack the necessary infrastructure and equipment to ensure the safe and sterile administration of the drug. Additionally, the individuals performing the injections may not have the required medical training or expertise, further increasing the risk to patients.
Furthermore, the origins of counterfeit Botox are often unclear. In the Tennessee cases, the injections were linked to products with unknown sources, strongly suggesting that they were counterfeit. This lack of transparency regarding the manufacturing and distribution of the product raises serious concerns about its safety and efficacy.
Another issue with counterfeit Botox is the potential for substitution or adulteration of the product. Since these counterfeit products are not subject to the same regulatory oversight as genuine Botox, there is a higher likelihood of them being contaminated or diluted with other substances. This not only compromises the effectiveness of the treatment but also increases the risk of adverse reactions and complications.
Moreover, the individuals administering counterfeit Botox may not be aware of the appropriate dosage or injection techniques. This lack of knowledge and experience can lead to improper administration, resulting in ineffective results or even serious medical complications.
Given the dangers associated with counterfeit Botox, it is crucial for individuals seeking cosmetic procedures to ensure that they receive treatment from licensed medical professionals in reputable medical facilities. These professionals have the necessary training, expertise, and access to genuine Botox, ensuring the safety and effectiveness of the procedure.
Symptoms and Risks
Early symptoms of botulism can include double or blurred vision, drooping eyelids, slurred speech, difficulty swallowing, dry mouth, and difficulty breathing. If left untreated, the toxin can lead to full paralysis, including muscles used for breathing. The affected individuals in both Tennessee and Illinois experienced similar symptoms, such as blurred or double vision, droopy face, fatigue, shortness of breath, difficulty breathing, and a hoarse voice, after receiving their injections.
Health officials in Illinois have issued a warning, urging residents to exercise caution when considering cosmetic treatments. They emphasize the importance of receiving these treatments only from licensed professionals who use FDA-approved products. If anyone experiences health problems after a recent cosmetic treatment, they are advised to contact their healthcare provider immediately for assistance.
It is crucial to prioritize safety and ensure that all cosmetic procedures are performed in authorized settings by qualified professionals. The current outbreak serves as a reminder of the potential risks associated with counterfeit products and unapproved practices.
Furthermore, it is essential to be aware of the risks involved in cosmetic treatments. While botulinum toxin injections are generally safe when administered correctly, there have been cases where individuals have suffered adverse effects due to counterfeit or improperly administered products. In some instances, unlicensed individuals may offer cosmetic treatments at a lower cost, attracting unsuspecting individuals who are unaware of the potential dangers.
These counterfeit products may not undergo the same rigorous testing and quality control as FDA-approved products. As a result, they can contain impurities or incorrect concentrations of the toxin, leading to serious health complications. In the case of the recent outbreak in Tennessee and Illinois, it is suspected that the affected individuals received injections containing a contaminated or counterfeit botulinum toxin.
The risks associated with these unapproved practices extend beyond the immediate health effects. In addition to the physical harm they can cause, individuals who undergo cosmetic treatments from unlicensed providers may also face legal consequences. The unauthorized administration of botulinum toxin injections is a violation of medical regulations and can result in criminal charges for those involved.
Therefore, it is crucial for individuals considering cosmetic treatments to do their due diligence and research the providers thoroughly. They should verify the credentials and qualifications of the professionals offering the treatments and ensure that they are authorized to perform the procedures. Additionally, individuals should inquire about the products being used and confirm that they are FDA-approved.
By taking these precautions, individuals can protect themselves from the potential risks associated with counterfeit products and unapproved practices. It is essential to prioritize safety and make informed decisions when it comes to cosmetic treatments, as the consequences of negligence can be severe.